(Updated to include USCCB statement.)
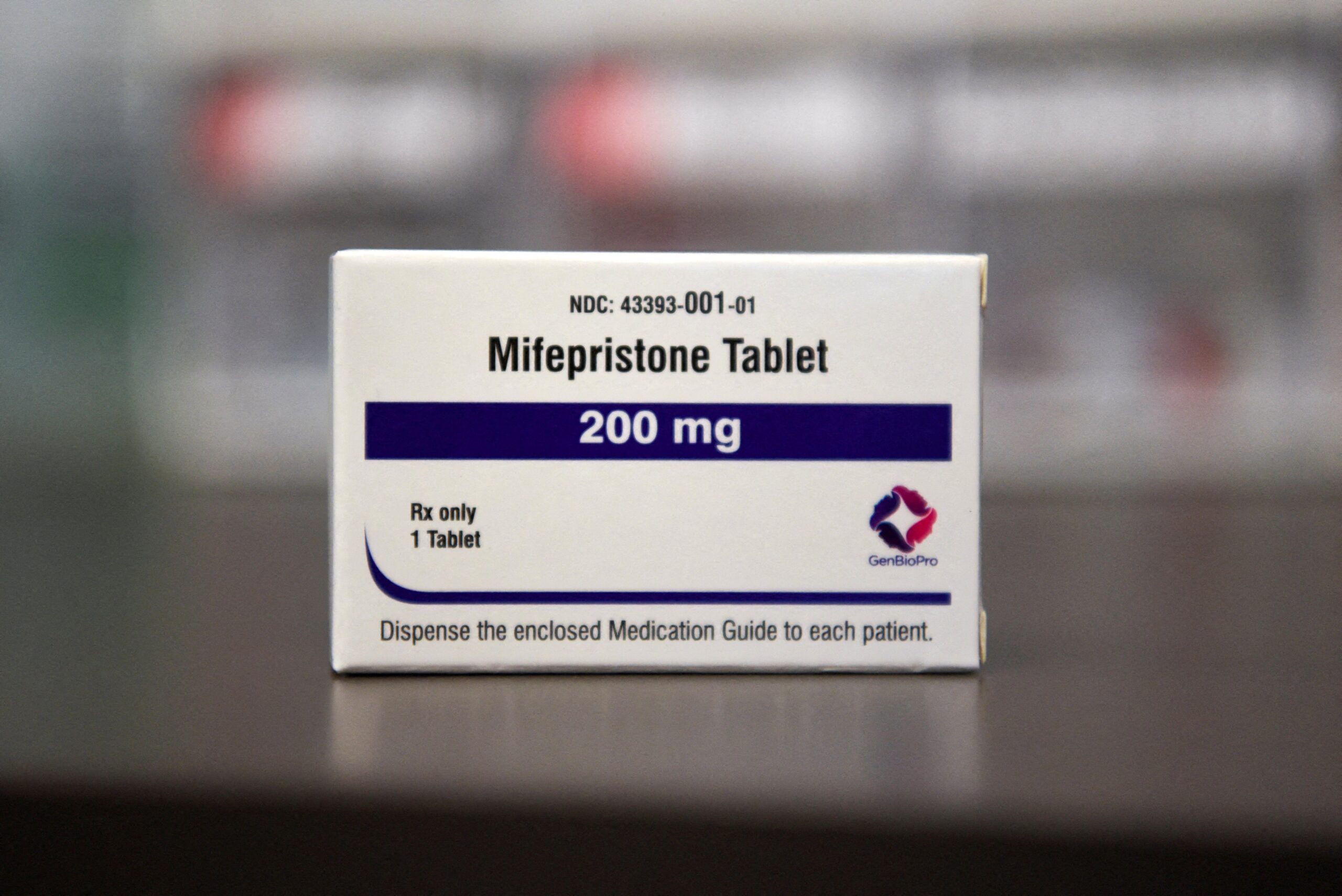
WASHINGTON (OSV News) — The U.S. Supreme Court said April 21 it would block a lower court’s restrictions on an abortion pill, leaving the drug on the market while litigation over the drug proceeds.
The court’s order was an apparent 7-2 vote, with Justices Clarence Thomas and Samuel Alito publicly dissenting.
The decision froze a lower court’s ruling to stay the U.S. Food and Drug Administration’s approval of the drug. The Justice Department and a pharmaceutical company that manufactures the abortion pill mifepristone previously asked the Supreme Court to intervene in the case after an appeals court allowed portions of the ruling by U.S. District Judge Matthew Kacsmaryk to take effect.
A coalition of pro-life opponents of mifepristone, the first of two drugs used in a medication or chemical abortion, had filed suit in an effort to revoke the FDA’s approval of the drug, arguing the government violated its own safety standards when it first approved the drug in 2000. However, proponents argued mifepristone poses statistically little risk to women using it for abortion early in pregnancy, and claim the drug is being singled out for political reasons.
In an April 21 statement, President Joe Biden said he would continue “to stand by FDA’s evidence-based approval of mifepristone, and my Administration will continue to defend FDA’s independent, expert authority to review, approve, and regulate a wide range of prescription drugs.”
On April 22, Bishop Michael F. Burbidge of Arlington, chairman of the U.S. Conference of Catholic Bishops’ Committee on Pro-Life Activities, called the Supreme Court’s interim order “a tremendous disappointment, both for the loss of innocent preborn life from chemical abortion, and for the danger that chemical abortion poses to women.”
(For the full OSV News story, see your local Catholic news source.)